Aqueous solution and non aquesous solution | concentrated solution and dilute solution | Binary solution and Ternary solution
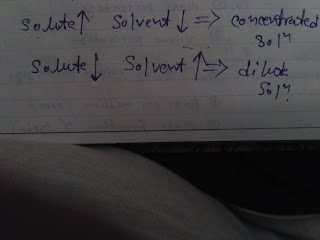
Aqueous solution : Those solution in which water is present as solvent, then the solution is known as aqueous solution. Non - Aqueous solution : Those solution in which water is not present as a solvent, then the solution is known as Non - Aqueous solution. Concentrated solution : Those solution in whitch large amount of solute dissolved in given solvent , then the solution is known as concentrated solution. Dilute solution : Those solution in which small amount of solute dissolved in large amount of solvent, then the solution is known as dilute solution. Binary solution : Homogeneous mixture of two substances is known as binary solution. Example : glucose and water Ternary solution : Homogeneous mixture of three substances is known as ternary solution. Example : Sugar + salt + water
